Should First Lady Michele Obama Get Involved With Breast Cancer
Houston ( AP )The Pharmaceutical Lobbyist is trying to stop The Breast cancer Cure. Just as we are on the door step to a cure we now have a wave of science reports trying to make genomics
science too broad. Roche Names Lufthansa CEO Franz to Replace their present CEO Humer. " Their Stock Price Drops" In the New York Times Today An Article on Mosaicism . Mosaicism by definition is the presence of two or more populations of cells with different genotypes in one individual. We contain genetic multitudes. Not long ago, researchers had thought it was rare for the cells in a single healthy person to differ genetically in a significant way. But scientists are finding that it’s quite common for an individual to have multiple genomes. Some people, for example, have groups of cells with mutations that are not found in the rest of the body. Some have genomes that came from other people. “There have been whispers in the matrix about this for years, even decades, but only in a very hypothetical sense,” said Alexander Urban, Brought To You By The Dalmore Single Malt a geneticist at Stanford University. Even three years ago, suggesting that there was widespread genetic variation in a single body would have been met with skepticism, he said. “You would have just run against the wall.”But a series of recent papers by Dr. Urban and others has demonstrated that those whispers were not just hypothetical. The variation in the genomes found in a single
person is too large to be ignored. “We now know it’s there,” Dr. Urban said. “Now we’re mapping this new continent.”NIH In Nature Journal talk about how Francis Collins and other are making President Obama BRAIN Initiative goals for 2014 too broad. The NIH is one of three government partners in the BRAIN Initiative, and the first to announce research goals; the report will guide the agency's spending of the $40 million it has committed for 2014. The Defense Advanced Research Projects Agency in Arlington,
Virginia, has said that it does not intend to release a road map for its $50-million contribution to the project, and the National Science Foundation, also in Arlington, has not yet finalized plans for its $20-million commitment in the next year.Fifteen leading neuroscientists selected by Collins — nicknamed the 'Dream Team' — prepared the NIH plan after holding four workshops around the country since May. The resulting report calls for molecular characterization of cell types in the brain, with
the eventual goal of manipulating specific neuronal populations of interest. It says that better diagrams of neural circuits are needed to accompany the deluge of data that will be enabled by large-scale recording techniques. The NIH committee also recommends the development of theoretical and computational approaches to deal with the massive data sets that such research is expected to produce.We need to stay focus on the breast cancer cure prize In the Houston Chronicle Today
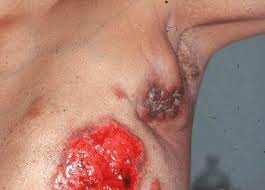
Lori Sumako received her second cancer diagnosis in October 2007, when her doctor suggested she simply enjoy what time she had left. Sumako looked at her in disbelief, and vowed to find a new oncologist.She and husband Rick Mitchell wandered into a breast cancer conference a few days later, and Sumako spotted a man wearing a suit and a stethoscope. Yes, he was an oncologist, and he told her exactly what she needed to hear. “I don’t believe in palliative care,” Dr. Khaled Jabboury said to her. “The goal is complete remission.”
As Sumako and Jabboury talked, Mitchell interviewed radiation oncologist Dr. Karl King. The two physicians became the leaders of Sumako’s cancer-fighting team. First came chemotherapy, a cocktail of six drugs. One had to be discontinued because of painful side effects. But the others worked so well that her small tumors disappeared. That left one, which was blasted into nothingness by radio-frequency ablation. Then came four months of radiation.
Early phase of biomarker discovery in three clinically important types of breast cancer using a panel of human cell lines: HER2 positive, hormone receptor positive and HER2 negative, and triple negative (HER2-, ER-, PR-). We identified and characterized the most abundant secreted, sloughed, or leaked proteins released into serum free media from these breast cancer cell lines using a combination of protein fractionation methods before LC-MS/MS mass spectrometry analysis.
A total of 249 proteins were detected in the proximal fluid of 7 breast cancer cell lines. The expression of a selected group of high abundance and/or breast cancer-specific potential biomarkers including thromobospondin 1, galectin-3 binding protein, cathepsin D, vimentin, zinc-α2-glycoprotein, CD44, and EGFR from the breast cancer cell lines and in their culture media were further validated by Western blot analysis. Interestingly, mass spectrometry identified a cathepsin D protein single-nucleotide polymorphism (SNP) by alanine to valine replacement from the MCF-7 breast cancer cell line. Comparison of each cell line media proteome displayed unique and consistent biosignatures regardless of the individual group classifications, demonstrating the potential for stratification of breast cancer. On the basis of the cell line media proteome, predictive Tree software was able to categorize each cell line as HER2 positive, HER2 negative, and hormone receptor positive and triple negative based on only two proteins, muscle fructose 1,6-bisphosphate aldolase and keratin 19. In addition, the predictive Tree software clearly identified MCF-7 cell line overexpresing the HER2 receptor with the SNP cathepsin D biomarker.Approximately 30% of malignant breast cancers demonstrate overamplification of the human epidermal receptor type 2 (HER2) gene. HER-2 can be resistant to low-doses of anthracycline-based chemotherapy. The Good News is that science has advanced. Sections of microarray provide targets for parallel in situ detection of DNA, RNA and protein targets in each specimen on the array. The better News is that Genomics is on the Clock. Genomics provide a faster cheaper more effective way to detect the Her2 gene by using Semiconductor Sequencing. A example of this technique is Gennxeix Biotech Inc Semiconductor Sequencing. "Quantum Theory" In Action for Breast Cancer Patients. One of the major player and touch down makers for breast cancer is Houston Texas Methodist Hospital. In A clinical Trial A Rev. Noel Denison, a retired Methodist minister, was diagnosed with locally advanced HER2-positive breast cancer and is enrolled in the study at Methodist, one of only two locations in the United States. The clinical trial is for locally advanced or metastatic HER2-positive breast cancer and combines standard chemotherapy with trastuzumab emtansine, better known in the breast cancer world as T-DM1, and pertuzumab,a monoclonal antibody that also attaches to HER2 on cancer cells. Using
Genomics and semiconductors to detect breast cancer plus T-DM1 to treat breast cancer is a winning combination. What is T-DM1? T-DM1 is in a new class of cancer-fighting agents called antibody drug conjugates. By combining the antibody trastuzumab directly with docetaxel (standard chemotherapy) and/or pertuzumab, the T-DMI is designed to attack the tumor cells directly and deliver the chemotherapy. Trastuzumab emtansine (T-DM1) consists of our proprietary DM1 cancer-killing agent attached to the HER2-binding antibody, trastuzumab, developed by Genentech (a member of the Roche Group)The Science and Medical communities must stay focus on the gifts that genomic science has given us.